In this article, I will showcase two compelling use cases demonstrating the benefits and potential of AI agents in the development of medical devices.
The regulated environments for medical devices are increasingly complex, implying costly efforts and long timelines before and after market launch.
In Europe, the EU Medical Device Regulation (MDR) was adopted in 2017, promising transparency and traceability. In a previous post, we highlighted that despite significant investments, the transparency promised through the Eudamed database has not yet been realized.
Modern AI agents can come to the rescue. With careful selection of use cases and proper implementation, the corresponding effort can be drastically reduced, timelines shortened, and data quality greatly improved.
There are dozens of relevant use cases to be explored and adapted to different industrial actors’ organizations and processes.
Example 1: Navigating Legislation Using AI RAG Agents
The EU MDR legislation is not only 175 pages long but also accompanied by dozens of guidance and Q&A documents. Familiarizing yourself with and finding the relevant sections for different functions is a daunting task.
Large Language Model (LLM) chat interfaces (e.g., Gemini and ChatGPT) have been trained on this data as it has been public since 2017, offering great help out of the box.
Building your own Retrieval-Augmented Generation (RAG) AI agent provides significant improvements. With this approach, you gain further control over the exact version of each legislation, receive sources for generated answers, and incorporate company-specific data (such as internal guidance and regulatory communications), quality assurance, and monitoring of the agent.
Moreover, you can customize the user interface and flow to best suit specific users.
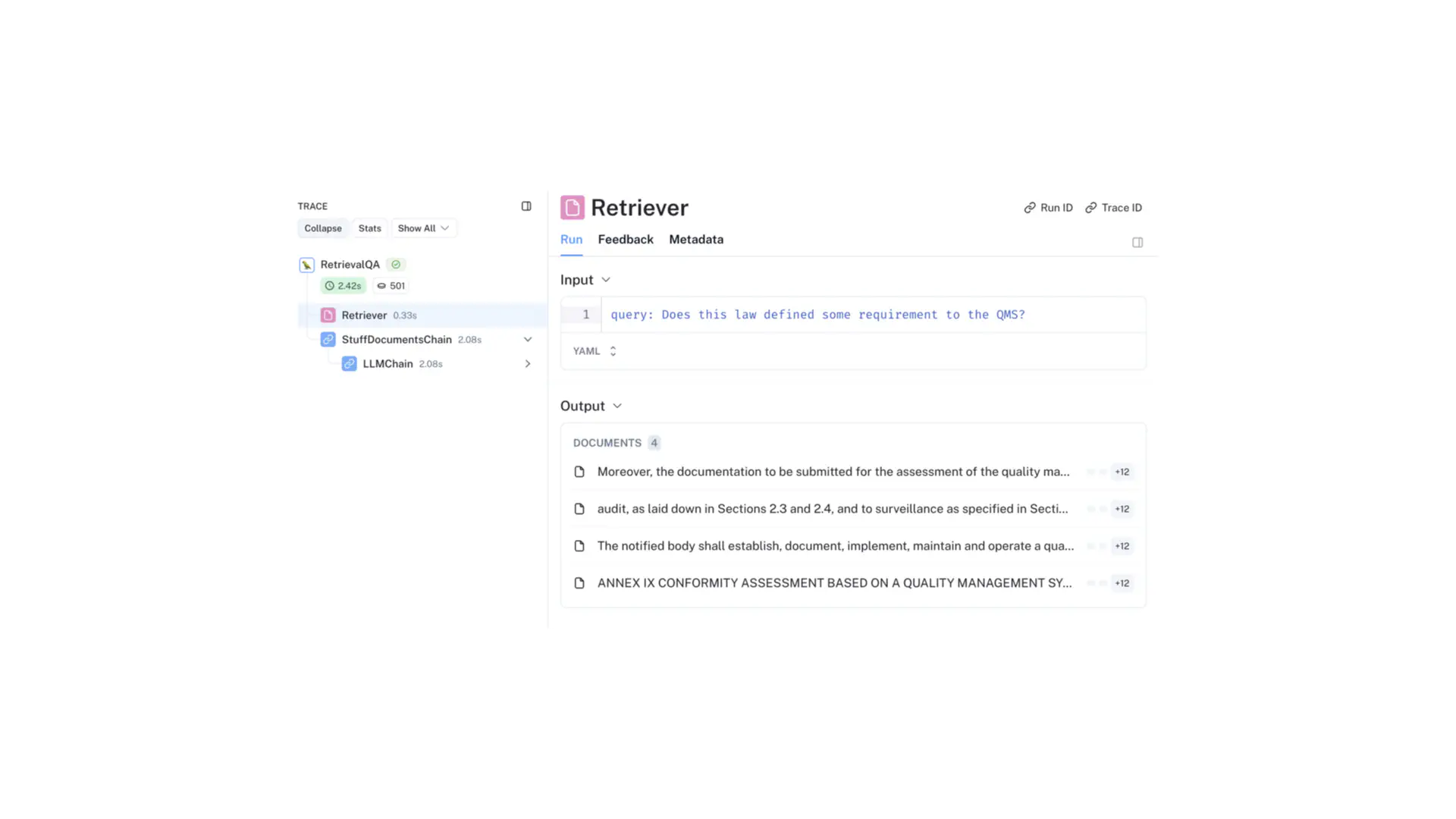
I have built a RAG agent using the LangChain framework to illustrate feasibility and benefits. The data includes the EU MDR acts and guidance documents. The agent looks up the relevant sections of these documents, provides them as sources, and uses LLMs to deliver interpretable answers.
Monitoring traces of some queries illustrate the process, showing how relevant data is retrieved.
Example 2: Preparing High-Quality Evidence by Extracting EU MDR Data from Documents
AI agents are not limited to question-and-answer tasks; they can also transform work process flows.
Legislation requires the provision of high-quality, curated, and well-formatted data.
AI agents can automatically extract information, perform quality checks, and format it as required.
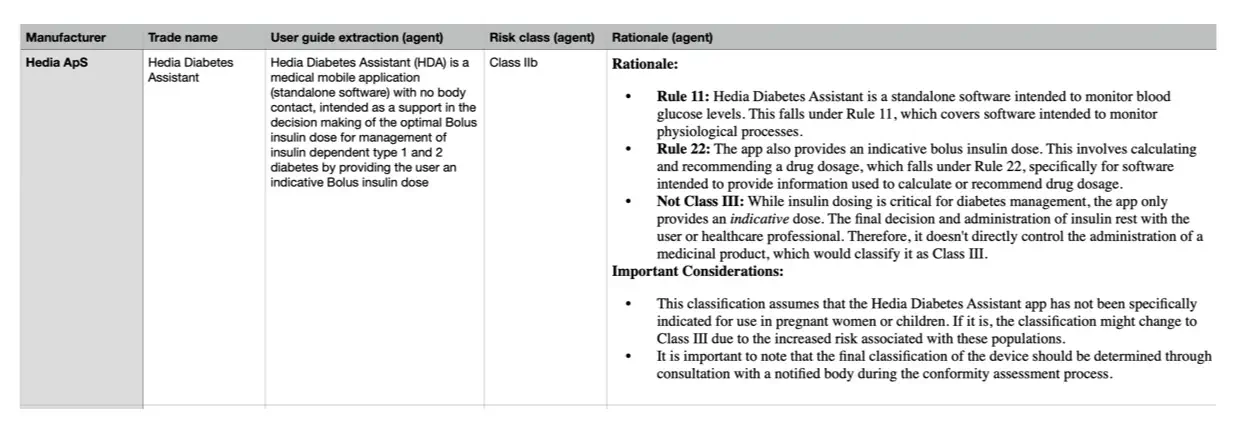
I have developed an MDR risk classification AI agent to demonstrate this concept. Based on user guide information, the AI agent classifies devices or applications using MDR-defined rules and provides a rationale.
This agent helps prepare an overview of all devices and applications within a company and market. It optimizes workflows through automatic data augmentation and checks.
In the context of EU MDR, it supports the development of a relevant Eudamed database by leveraging LLMs for function calling, generating structured outcomes, and integrating functionality into an ETL pipeline.
The figure illustrates the outcome generated by the agent for a specific SaMD product.
Conclusion
AI agents can dramatically improve the efficiency of actors in the medical device industry. This requires active involvement from industrial actors in understanding the tools’ potential, refining and implementing use cases with a structured approach.
Such a transformation involves changes in workflows and processes. Now is the time to engage in this transformative change. It is a positive shift, bringing clear sociological benefits through a more efficient, safer, and transparent medical device and software as a medical device industry.