The Driving Forces: Regulatory Compliance and Business Differentiation
In the medical device industry, effective complaint handling and post-market surveillance (PMS) are not just good practice, they’re essential. Here’s why:
Regulatory Compliance: Meeting stringent regulations like the EU MDR and FDA 21 CFR Part 820 is a must. Robust complaint and PMS systems are crucial for demonstrating compliance and avoiding costly regulatory actions.
Patient Safety: Customer complaints offer valuable insights into potential safety issues. Timely response and thorough investigation are vital to protect patients and mitigate risks.
Continuous Improvement: Complaint and PMS data are a treasure trove of information. Analyzing this data fuels continuous improvement, leading to safer, more effective medical devices.
Commercial Success: Effective complaint handling and PMS build trust and enhance your brand’s reputation. They also reduce product liability risks, provide valuable market intelligence, and give you a competitive edge.
Dive Deeper: The Business Impact and Our Approach
Want to learn more about the strategic importance of service recovery and our proven methodology?
Ready to Take Action?
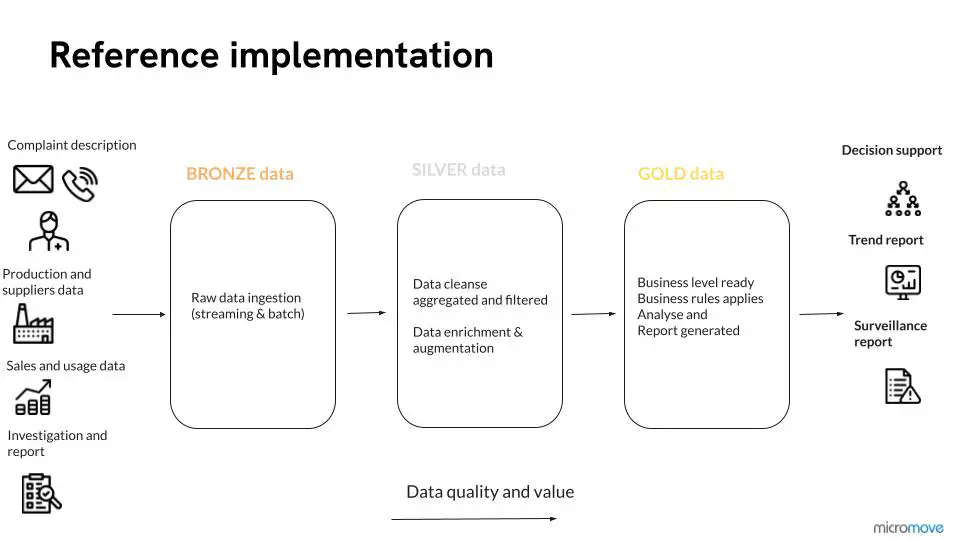
Contact us today for a comprehensive overview of our reference implementation (illustrated below).